
Back Asymetrická indukce Czech Asymmetrische Induktion German Inducción asimétrica Spanish Asymmetrische inductie Dutch Indukcja asymetryczna Polish Indução assimétrica Portuguese Правило Крама Russian Правило Крама Ukrainian 不对称诱导 Chinese
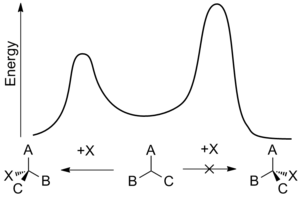
Asymmetric induction (also enantioinduction) describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment.[1] Asymmetric induction is a key element in asymmetric synthesis.
Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates.[2] Several types of induction exist.
Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.[clarification needed]