
Back أتروبين Arabic Antropin Azerbaijani آتروپین AZB Атрапін Byelorussian Атропин Bulgarian অ্যাট্রোপিন Bengali/Bangla Atropin BS Atropina Catalan Atropin Czech Atropin Welsh
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Atropen, others |
| Other names | Daturin[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682487 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, intramuscular, rectal, ophthalmic |
| Drug class | antimuscarinic (anticholinergic) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 25% |
| Metabolism | ≥50% hydrolysed to tropine and tropic acid |
| Onset of action | c. 1 minute[5] |
| Elimination half-life | 2 hours |
| Duration of action | 30 to 60 min[5] |
| Excretion | 15–50% excreted unchanged in urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.000.096 |
| Chemical and physical data | |
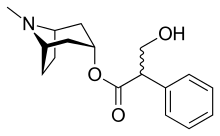
| Formula | C17H23NO3 |
| Molar mass | 289.375 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery.[6] It is typically given intravenously or by injection into a muscle.[6] Eye drops are also available which are used to treat uveitis and early amblyopia.[7][8] The intravenous solution usually begins working within a minute and lasts half an hour to an hour.[5] Large doses may be required to treat some poisonings.[6]
Common side effects include dry mouth, abnormally large pupils, urinary retention, constipation, and a fast heart rate.[6] It should generally not be used in people with closed-angle glaucoma.[6] While there is no evidence that its use during pregnancy causes birth defects, this has not been well studied so sound clinical judgment should be used.[9] It is likely safe during breastfeeding.[9] It is an antimuscarinic (a type of anticholinergic) that works by inhibiting the parasympathetic nervous system.[6]
Atropine occurs naturally in a number of plants of the nightshade family, including deadly nightshade (belladonna), Jimson weed, and mandrake.[10] It was first isolated in 1833,[11] It is on the World Health Organization's List of Essential Medicines.[12] It is available as a generic medication.[6][13][14]
- ^ Rafinesque CS (1828). Medical Flora; Or, Manual of the Medical Botany of the United States of ... - Constantine Samuel Rafinesque - Internet Archive. Atkinson & Alexander. p. 148. Retrieved 2012-11-07.
- ^ "AusPAR: Atropine sulfate monohydrate". Therapeutic Goods Administration (TGA). 31 May 2022. Archived from the original on 31 May 2022. Retrieved 12 June 2022.
- ^ Cite error: The named reference
Atropine sulfate FDA labelwas invoked but never defined (see the help page). - ^ "Atropine- atropine sulfate solution/ drops". DailyMed. 22 February 2022. Archived from the original on 16 March 2022. Retrieved 16 March 2022.
- ^ a b c Barash PG (2009). Clinical anesthesia (6th ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. p. 525. ISBN 9780781787635. Archived from the original on 2015-11-24.
- ^ a b c d e f g "Atropine". The American Society of Health-System Pharmacists. Archived from the original on 2015-07-12. Retrieved Aug 13, 2015.
- ^ Hamilton RJ, Duffy AN, Stone D, Spencer A (2014). Tarascon pharmacopoeia (15 ed.). Jones & Bartlett Publishers. p. 386. ISBN 9781284056716. Archived from the original on 2015-10-02.
- ^ "Amblyopia (Lazy Eye)". National Eye Institute. 2019-07-02. Archived from the original on 2020-01-31. Retrieved 2020-01-31.
Putting special eye drops in the stronger eye. A once-a-day drop of the drug atropine can temporarily blur near vision, which forces the brain to use the other eye. For some children, this treatment works as well as an eye patch, and some parents find it easier to use (for example, because young children may try to pull off eye patches).
- ^ a b "Atropine Pregnancy and Breastfeeding Warnings". Archived from the original on 6 September 2015. Retrieved 14 August 2015.
- ^ Brust JC (2004). Neurological aspects of substance abuse (2 ed.). Philadelphia: Elsevier. p. 310. ISBN 9780750673136. Archived from the original on 2015-10-02.
- ^ Ainsworth S (2014). Neonatal Formulary: Drug Use in Pregnancy and the First Year of Life. John Wiley & Sons. p. 94. ISBN 9781118819593. Archived from the original on 2015-10-02.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Hamilton RJ (2014). Tarascon pharmacopoeia (15 ed.). Jones & Bartlett Publishers. p. 386. ISBN 9781284056716. Archived from the original on 2015-10-02.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.