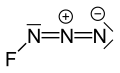Back Fluorazid Czech Fluorazid German فلوئور آزید Persian Azoture de fluor French Fluorin azida ID Fluoruro di triazoto Italian Азид фтора Russian Fluor azid Serbian புளோரின் அசைடு Tamil 叠氮化氟 Chinese
| |||
| Names | |||
|---|---|---|---|
| Other names
triazadienyl fluoride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| FN3 | |||
| Molar mass | 61.019 g/mol | ||
| Appearance | Yellow-green gas | ||
| Melting point | −139 °C (−218 °F; 134 K) | ||
| Boiling point | −30 °C (−22 °F; 243 K) | ||
| Explosive data | |||
| Shock sensitivity | Extreme | ||
| Friction sensitivity | Extreme | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Extremely sensitive explosive | ||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Other cations
|
Hydrazoic acid Chlorine azide Bromine azide Iodine azide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3.[1] Its properties resemble those of ClN3, BrN3, and IN3.[2] The bond between the fluorine atom and the nitrogen is very weak, leading to this substance being very unstable and prone to explosion.[3] Calculations show the F–N–N angle to be around 102° with a straight line of 3 nitrogen atoms.[4]
The gas boils at –30° and melts at –139 °C.[5]
It was first made by John F. Haller in 1942.[6]
- ^ Gipstein, Edward; John F. Haller (1966). "Absorption Spectrum of Fluorine Azide". Applied Spectroscopy. 20 (6): 417–418. Bibcode:1966ApSpe..20..417G. doi:10.1366/000370266774386470. ISSN 0003-7028. S2CID 96337253.
- ^ Saxena, P. B. (2007-01-01). Chemistry of Interhalogen Compounds. Discovery Publishing House. p. 96. ISBN 9788183562430. Retrieved 16 June 2014.
- ^ Rademacher, Paul; Andreas J. Bittner; Gabriele Schatte; Helge Willner (1988). "Photoelectron Spectrum and Electronic Structure of Triazadienyl Fluoride, N3F". Chemische Berichte. 121 (3): 555–557. doi:10.1002/cber.19881210325. ISSN 0009-2940.
- ^ Peters, Nancy J. S.; Leland C. Allen; Raymond A. Firestone (1988). "Fluorine azide and fluorine nitrate: structure and bonding". Inorganic Chemistry. 27 (4): 755–758. doi:10.1021/ic00277a035. ISSN 0020-1669.
- ^ Cite error: The named reference
Gholivand1987was invoked but never defined (see the help page). - ^ Lowe, Derek (21 October 2008). "Things I Won't Work With: Triazadienyl Fluoride". In the Pipeline. Retrieved 15 June 2014.