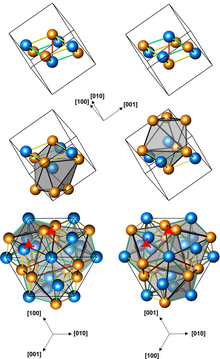
 Structures of left-handed and right-handed FeGe crystals (3 presentations, with different numbers of atoms per unit cell; orange atoms are Ge)
| |
| Names | |
|---|---|
| IUPAC name
Iron germanide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| FeGe | |
| Molar mass | 128.47 g/mol |
| Structure | |
| Cubic[1] | |
| P213 (No. 198), cP8 | |
a = 0.4689 nm
| |
Formula units (Z)
|
4 |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Iron silicide |
Other cations
|
Manganese germanide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Iron germanide (FeGe) is an intermetallic compound, a germanide of iron. At ambient conditions it crystallizes in three polymorphs with monoclinic, hexagonal and cubic structures. The cubic polymorph has no inversion center, it is therefore helical, with right-hand and left-handed chiralities.[1]
