 MnSi prepared by zone melting
| |
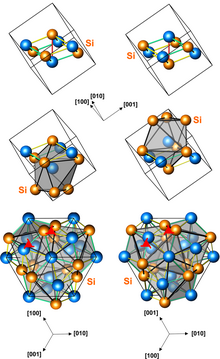 Structures of left-handed and right-handed MnSi crystals (3 presentations, with different numbers of atoms per unit cell)
| |
| Names | |
|---|---|
| IUPAC name
Manganese silicide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| MnSi | |
| Molar mass | 83.023 g/mol |
| Melting point | 1,280 °C (2,340 °F; 1,550 K)[2] |
| 31.3×10−6 emu/g[1] | |
| Thermal conductivity | 0.1 W/(cm·K)[2] |
| Structure | |
| Cubic[3] | |
| P213 (No. 198), cP8 | |
a = 0.45598(2) nm
| |
Formula units (Z)
|
4 |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Manganese germanide |
Other cations
|
Iron silicide Cobalt silicide |
Related compounds
|
Manganese disilicide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Manganese monosilicide (MnSi) is an intermetallic compound, a silicide of manganese. It occurs in cosmic dust as the mineral brownleeite. MnSi has a cubic crystal lattice with no inversion center; therefore its crystal structure is helical, with right-hand and left-hand chiralities.
MnSi is a paramagnetic metal that turns into a ferromagnet at cryogenic temperatures below 29 K. In the ferromagnetic state, the spatial arrangement of electron spins in MnSi changes with magnetic field, forming helical, conical, skyrmion, and regular ferromagnetic phases.
- ^ Shinoda, Daizaburo; Asanabe, Sizuo (1966). "Magnetic Properties of Silicides of Iron Group Transition Elements". Journal of the Physical Society of Japan. 21 (3): 555. Bibcode:1966JPSJ...21..555S. doi:10.1143/JPSJ.21.555.
- ^ a b Levinson, Lionel M. (1973). "Investigation of the defect manganese silicide MnnSi2n−m". Journal of Solid State Chemistry. 6 (1): 126–135. Bibcode:1973JSSCh...6..126L. doi:10.1016/0022-4596(73)90212-0.
- ^ Cite error: The named reference
r1was invoked but never defined (see the help page).
