
Back Potentialhyperfläche German Superficie de energía potencial Spanish Energia potentzial gainazala Basque Surface d'énergie potentielle French Superficie di energia potenziale Italian ポテンシャルエネルギー曲面 Japanese Поверхность потенциальной энергии Russian Potansiyel enerji yüzeyi Turkish Поверхня потенціальної енергії Ukrainian 势能面 Chinese
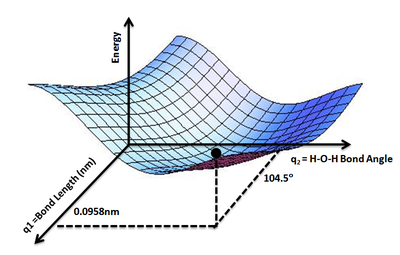

A potential energy surface (PES) or energy landscape describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates; if there is only one coordinate, the surface is called a potential energy curve or energy profile. An example is the Morse/Long-range potential.
It is helpful to use the analogy of a landscape: for a system with two degrees of freedom (e.g. two bond lengths), the value of the energy (analogy: the height of the land) is a function of two bond lengths (analogy: the coordinates of the position on the ground).[1]
The PES concept finds application in fields such as physics, chemistry and biochemistry, especially in the theoretical sub-branches of these subjects. It can be used to theoretically explore properties of structures composed of atoms, for example, finding the minimum energy shape of a molecule or computing the rates of a chemical reaction. It can be used to describe all possible conformations of a molecular entity, or the spatial positions of interacting molecules in a system, or parameters and their corresponding energy levels, typically Gibbs free energy. Geometrically, the energy landscape is the graph of the energy function across the configuration space of the system. The term is also used more generally in geometric perspectives to mathematical optimization, when the domain of the loss function is the parameter space of some system.
- ^ Potential-energy (reaction) surface in Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997)