
Back ريتونافير Arabic ریتوناویر AZB Ritonavir Catalan Ritonafir Welsh Ritonavir German Ritonavir Esperanto Ritonavir Spanish Erritonabir Basque ریتوناویر Persian Ritonaviiri Finnish
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /rɪˈtɒnəˌvɪər/ rih-TO-nə-veer |
| Trade names | Norvir |
| Other names | RTV |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696029 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 98–99% |
| Metabolism | Liver |
| Elimination half-life | 3–5 hours |
| Excretion | Mostly fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.125.710 |
| Chemical and physical data | |
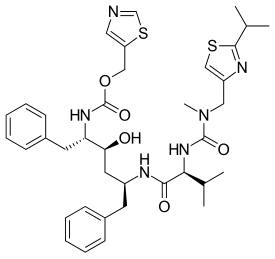
| Formula | C37H48N6O5S2 |
| Molar mass | 720.95 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ritonavir, sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS.[4][5][6] This combination treatment is known as highly active antiretroviral therapy (HAART).[6] Ritonavir is a protease inhibitor, though it now mainly serves to boost the potency of other protease inhibitors.[6][7] It may also be used in combination with other medications to treat hepatitis C and COVID-19.[8][9] It is taken by mouth.[6] Tablets of ritonavir are not bioequivalent to capsules, as the tablets may result in higher peak plasma concentrations.[6]
Common side effects of ritonavir include nausea, vomiting, loss of appetite, diarrhea, and numbness of the hands and feet.[6] Serious side effects include liver complications, pancreatitis, allergic reactions, and arrythmias.[6] Serious interactions may occur with a number of other medications including amiodarone and simvastatin.[6] At low doses, it is considered to be acceptable for use during pregnancy.[10] Ritonavir is of the protease inhibitor class.[6] However, it is also commonly used to inhibit the enzyme that metabolizes other protease inhibitors.[11] This inhibition allows lower doses of these latter medications to be used.[11]
Ritonavir was patented in 1989 and came into medical use in 1996.[12][13] It is on the World Health Organization's List of Essential Medicines.[14][15] Ritonavir capsules were approved as a generic medication in the United States in 2020.[16]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved October 22, 2023.
- ^ "Notice: Nirmatrelvir (COVID-19) added to Prescription Drug List (PDL)". Health Canada. January 17, 2022. Archived from the original on May 29, 2022. Retrieved June 25, 2022.
- ^ "Norvir Product information". Health Canada. April 25, 2012. Retrieved June 25, 2022.
- ^ a b "Norvir- ritonavir tablet, film coated Norvir- ritonavir solution Norvir- ritonavir powder". DailyMed. Archived from the original on November 18, 2021. Retrieved November 17, 2021.
- ^ a b Cite error: The named reference
Norvir EPARwas invoked but never defined (see the help page). - ^ a b c d e f g h i "Ritonavir". The American Society of Health-System Pharmacists. Archived from the original on October 17, 2015. Retrieved October 23, 2015.
- ^ Talha B, Dhamoon AS (August 8, 2023). "Ritonavir". StatPearls. StatPearls Publishing. PMID 31335032. Retrieved January 6, 2024.
- ^ Cite error: The named reference
FDA-ucm427530was invoked but never defined (see the help page). - ^ Akinosoglou K, Schinas G, Gogos C (November 2022). "Oral Antiviral Treatment for COVID-19: A Comprehensive Review on Nirmatrelvir/Ritonavir". Viruses. 14 (11): 2540. doi:10.3390/v14112540. PMC 9696049. PMID 36423149.
- ^ "Ritonavir Pregnancy and Breastfeeding Warnings". drugs.com. Archived from the original on September 7, 2015. Retrieved October 23, 2015.
- ^ a b British National Formulary 69 (69 ed.). Pharmaceutical Pr. March 31, 2015. p. 426. ISBN 9780857111562.
- ^ Hacker M (2009). Pharmacology principles and practice. Amsterdam: Academic Press/Elsevier. p. 550. ISBN 9780080919225. Archived from the original on June 17, 2020. Retrieved September 10, 2017.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 509. ISBN 9783527607495. Archived from the original on June 20, 2021. Retrieved August 27, 2020.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "First Generic Drug Approvals". U.S. Food and Drug Administration (FDA). Archived from the original on January 26, 2021. Retrieved February 13, 2021.