| |
| Names | |
|---|---|
| IUPAC name
Zinc(II) azide
| |
| Other names
Zinc diazide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
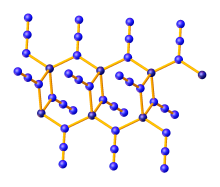
| Zn(N3)2 | |
| Molar mass | 149.4 g/mol |
| Appearance | White solid |
| Density | 2.559 g/cm3 (α polymorph) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Zinc azide Zn(N3)2 is an inorganic compound composed of zinc cations (Zn2+) and azide anions (N−3). It is a white, explosive solid that can be prepared by the protonolysis of diethylzinc with hydrazoic acid:[1]
- Zn(C2H5)2 + 2 HN3 → Zn(N3)2 + 2 C2H6
- ^ Schulz, Axel; Villanger, Alexander (2016). "Binary Zinc Azides". Chemistry: A European Journal. 22 (6): 2032–2038. doi:10.1002/chem.201504524. PMID 26749253.
