Back Butaan Afrikaans بوتان (كيمياء) Arabic Butanu AST Butan (maddə) Azerbaijani بوتان (هیدروکربون) AZB Бутан (рэчыва) Byelorussian Бутан (рэчыва) BE-TARASK Бутан (алкан) Bulgarian বিউটেন Bengali/Bangla Butan (plin) BS
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Butane[3] | |||
| Systematic IUPAC name
Tetracarbane (never recommended[3]) | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 969129 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.136 | ||
| EC Number |
| ||
| E number | E943a (glazing agents, ...) | ||
| 1148 | |||
| KEGG | |||
| MeSH | butane | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1011 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H10 | |||
| Molar mass | 58.124 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Gasoline-like or natural gas-like[1] | ||
| Density | 2.48 kg/m3 (at 15 °C (59 °F)) | ||
| Melting point | −140 to −134 °C; −220 to −209 °F; 133 to 139 K | ||
| Boiling point | −1 to 1 °C; 30 to 34 °F; 272 to 274 K | ||
| 61 mg/L (at 20 °C (68 °F)) | |||
| log P | 2.745 | ||
| Vapor pressure | ~170 kPa at 283 K [4] | ||
Henry's law
constant (kH) |
11 nmol Pa−1 kg−1 | ||
| −57.4·10−6 cm3/mol | |||
| Thermochemistry | |||
Heat capacity (C)
|
98.49 J/(K·mol) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−126.3–−124.9 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−2.8781–−2.8769 MJ/mol | ||
| Hazards[5] | |||
| GHS labelling: | |||

| |||
| Danger | |||
| H220 | |||
| P210 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −60 °C (−76 °F; 213 K) | ||
| 405 °C (761 °F; 678 K) | |||
| Explosive limits | 1.8–8.4% | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[1] | ||
REL (Recommended)
|
TWA 800 ppm (1900 mg/m3)[1] | ||
IDLH (Immediate danger)
|
1600 ppm[1] | ||
| Related compounds | |||
Related alkanes
|
|||
Related compounds
|
Perfluorobutane | ||
| Supplementary data page | |||
| Butane (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
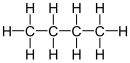
Butane (/ˈbjuːteɪn/) is an alkane with the formula C4H10. Butane exists as two isomers, n-butane with connectivity CH3CH2CH2CH3 and iso-butane with the formula (CH3)3CH. Both isomers are highly flammable, colorless, easily liquefied gases that quickly vaporize at room temperature and pressure. Butanes are a trace components of natural gases (NG gases). The other hydrocarbons in NG include propane, ethane, and especially methane, which are more abundant. Liquefied petroleum gas is a mixture of propane and some butanes.[6]
The name butane comes from the root but- (from butyric acid, named after the Greek word for butter) and the suffix -ane (for organic compounds).
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0068". National Institute for Occupational Safety and Health (NIOSH).
- ^ August Wilhelm Von Hofmann (1867). "I. On the action of trichloride of phosphorus on the salts of the aromatic monamines". Proceedings of the Royal Society of London. 15: 54–62. doi:10.1098/rspl.1866.0018. S2CID 98496840.
- ^ a b "General Principles, Rules, and Conventions". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. P-12.1. doi:10.1039/9781849733069-00001. ISBN 978-0-85404-182-4.
Similarly, the retained names 'ethane', 'propane', and 'butane' were never replaced by systematic names 'dicarbane', 'tricarbane', and 'tetracarbane' as recommended for analogues of silane, 'disilane'; phosphane, 'triphosphane'; and sulfane, 'tetrasulfane'.
- ^ W. B. Kay (1940). "Pressure-Volume-Temperature Relations for n-Butane". Industrial & Engineering Chemistry. 32 (3): 358–360. doi:10.1021/ie50363a016.
- ^ "Safety Data Sheet, Material Name: N-Butane" (PDF). USA: Matheson Tri-Gas Incorporated. 5 February 2011. Archived from the original (PDF) on 1 October 2011. Retrieved 11 December 2011.
- ^ Hammer, Georg; Lübcke, Torsten; Kettner, Roland; Pillarella, Mark R.; Recknagel, Herta; Commichau, Axel; Neumann, Hans-Joachim; Paczynska-Lahme, Barbara (2006). "Natural Gas". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a17_073.pub2. ISBN 978-3-527-30385-4.