
Back دكستروأمفيتامين Arabic دقزامفتامین AZB Декстроамфетамин Bulgarian Dextroamfetamin Czech Decstroamffetamin Welsh Dextroamphetamin German Dextroanfetamina Spanish Dextroanfetamina Basque دگزامفتامین Persian Deksamfetamiini Finnish
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌdɛkstroʊæmˈfɛtəmiːn/ |
| Trade names | Dexedrine, Zenzedi, Xelstrym, others |
| Other names | d-Amphetamine, (S)-Amphetamine, S(+)-Amphetamine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605027 |
| License data | |
| Pregnancy category |
|
| Dependence liability | Moderate[1][2] - high[3][4][5] |
| Addiction liability | Moderate[1][2] - high[3][4][5] |
| Routes of administration | By mouth, transdermal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: ~90%[13] |
| Protein binding | 15–40%[14] |
| Metabolism | CYP2D6,[19] DBH,[25] FMO3[26] |
| Onset of action | IR dosing: 0.5–1.5 hours[15][16] XR dosing: 1.5–2 hours[17][18] |
| Elimination half-life | 9–11 hours[19][20] pH-dependent: 7–34 hours[21] |
| Duration of action | IR dosing: 3–6 hours[17][22] XR dosing: 8–12 hours[23][17][22] |
| Excretion | Kidney (45%);[24] urinary pH-dependent |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.103 |
| Chemical and physical data | |
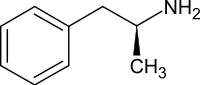
| Formula | C9H13N |
| Molar mass | 135.210 g·mol−1 |
| 3D model (JSmol) | |
| Density | 0.913 g/cm3 |
| Boiling point | 201.5 °C (394.7 °F) |
| Solubility in water | 20 |
| |
| |
| | |
Dextroamphetamine (INN:dexamfetamine) is a potent central nervous system (CNS) stimulant and enantiomer[note 1] of amphetamine that is prescribed for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy.[10][27] It is also used as an athletic performance and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant.
The amphetamine molecule exists as two enantiomers,[note 1] levoamphetamine and dextroamphetamine. Dextroamphetamine is the dextrorotatory, or 'right-handed', enantiomer and exhibits more pronounced effects on the central nervous system than levoamphetamine. Pharmaceutical dextroamphetamine sulfate is available as both a brand name and generic drug in a variety of dosage forms. Dextroamphetamine is sometimes prescribed as the inactive prodrug lisdexamfetamine dimesylate, which is converted into dextroamphetamine after absorption.
Dextroamphetamine, like other amphetamines, elicits its stimulating effects via several distinct actions: it inhibits or reverses the transporter proteins for the monoamine neurotransmitters (namely the serotonin, norepinephrine and dopamine transporters) either via trace amine-associated receptor 1 (TAAR1) or in a TAAR1 independent fashion when there are high cytosolic concentrations of the monoamine neurotransmitters[29] and it releases these neurotransmitters from synaptic vesicles via vesicular monoamine transporter 2.[30] It also shares many chemical and pharmacological properties with human trace amines, particularly phenethylamine and N-methylphenethylamine, the latter being an isomer of amphetamine produced within the human body.
- ^ a b Vitiello B (April 2008). "Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function". Child and Adolescent Psychiatric Clinics of North America. 17 (2): 459–74, xi. doi:10.1016/j.chc.2007.11.010. PMC 2408826. PMID 18295156.
- ^ a b Graham J, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Dittmann RW, et al. (January 2011). "European guidelines on managing adverse effects of medication for ADHD". European Child & Adolescent Psychiatry. 20 (1): 17–37. doi:10.1007/s00787-010-0140-6. eISSN 1435-165X. PMC 3012210. PMID 21042924.
- ^ a b Kociancic T, Reed MD, Findling RL (March 2004). "Evaluation of risks associated with short- and long-term psychostimulant therapy for treatment of ADHD in children". Expert Opinion on Drug Safety. 3 (2): 93–100. doi:10.1517/14740338.3.2.93. eISSN 1744-764X. PMID 15006715. S2CID 31114829.
- ^ a b Clemow DB, Walker DJ (September 2014). "The potential for misuse and abuse of medications in ADHD: a review". Postgraduate Medicine. 126 (5): 64–81. doi:10.3810/pgm.2014.09.2801. eISSN 1941-9260. PMID 25295651. S2CID 207580823.
- ^ a b Cite error: The named reference
Stahl's Essential Psychopharmacologywas invoked but never defined (see the help page). - ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Therapeutic Goods (Poisons Standard—February 2023) Instrument 2022". Australian Government Federal Register of Legislation. 26 September 2022. Retrieved 9 January 2023.
- ^ Fuller K (20 February 2022). "ADHD Stimulant Prescribing Regulations & Authorities in Australia & New Zealand". AADPA. Retrieved 9 January 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ a b "Dexedrine spansule- dextroamphetamine sulfate capsule, extended release". DailyMed. 10 January 2022. Retrieved 28 March 2022.
- ^ "Xelstrym- dextroamphetamine patch, extended release". DailyMed. 6 January 2023. Retrieved 21 January 2023.
- ^ "List of nationally authorised medicinal products : Active substance(s): dexamfetamine : Procedure No. PSUSA/00000986/202109" (PDF). Ema.europa.eu. Retrieved 5 June 2022.
- ^ Cite error: The named reference
handbook2022was invoked but never defined (see the help page). - ^ Cite error: The named reference
Drugbank-amphwas invoked but never defined (see the help page). - ^ Green-Hernandez C, Singleton JK, Aronzon DZ (1 January 2001). Primary Care Pediatrics. Lippincott Williams & Wilkins. p. 243. ISBN 978-0-7817-2008-3.|quote = Table 21.2 Medications for ADHD ... D-amphetamine ... Onset: 30 min.
- ^ "Dexedrine, ProCentra(dextroamphetamine) dosing, indications, interactions, adverse effects, and more". reference.medscape.com. Retrieved 4 October 2015.
Onset of action: 1–1.5 hr
- ^ a b c Millichap JG (2010). "Chapter 9: Medications for ADHD". In Millichap JG (ed.). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, USA: Springer. p. 112. ISBN 978-1-4419-1396-8.
Table 9.2 Dextroamphetamine formulations of stimulant medication
Dexedrine [Peak:2–3 h] [Duration:5–6 h] ...
Adderall [Peak:2–3 h] [Duration:5–7 h]
Dexedrine spansules [Peak:7–8 h] [Duration:12 h] ...
Adderall XR [Peak:7–8 h] [Duration:12 h]
Vyvanse [Peak:3–4 h] [Duration:12 h] - ^ Brams M, Mao AR, Doyle RL (September 2008). "Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder". Postgrad. Med. 120 (3): 69–88. doi:10.3810/pgm.2008.09.1909. PMID 18824827. S2CID 31791162.
Onset of efficacy was earliest for d-MPH-ER at 0.5 hours, followed by d, l-MPH-LA at 1 to 2 hours, MCD at 1.5 hours, d, l-MPH-OR at 1 to 2 hours, MAS-XR at 1.5 to 2 hours, MTS at 2 hours, and LDX at approximately 2 hours. ... MAS-XR, and LDX have a long duration of action at 12 hours postdose
- ^ a b Cite error: The named reference
FDA Pharmacokineticswas invoked but never defined (see the help page). - ^ "Adderall- dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablet". DailyMed. 27 February 2022. Retrieved 21 January 2023.
- ^ Cite error: The named reference
HSDB Toxnet October 2017 Full archived recordwas invoked but never defined (see the help page). - ^ a b Mignot EJ (October 2012). "A practical guide to the therapy of narcolepsy and hypersomnia syndromes". Neurotherapeutics. 9 (4): 739–752. doi:10.1007/s13311-012-0150-9. PMC 3480574. PMID 23065655.
- ^ Stahl SM (March 2017). "Amphetamine (D)". Prescriber's Guide: Stahl's Essential Psychopharmacology (6th ed.). Cambridge, United Kingdom: Cambridge University Press. pp. 39–44. ISBN 978-1-108-22874-9. Retrieved 8 August 2017.
- ^ "dextrostat (dextroamphetamine sulfate) tablet [Shire US Inc.]". DailyMed. Wayne, PA: Shire US Inc. August 2006. Retrieved 8 November 2013.
- ^ Lemke TL, Williams DA, Roche VF, Zito W (2013). Foye's Principles of Medicinal Chemistry (7th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 648. ISBN 978-1-60913-345-0.
Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ^ Cite error: The named reference
FMOwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Amph Useswas invoked but never defined (see the help page). - ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "enantiomer". doi:10.1351/goldbook.E02069
- ^ Cite error: The named reference
Millerwas invoked but never defined (see the help page). - ^ Cite error: The named reference
E Weihewas invoked but never defined (see the help page).
Cite error: There are <ref group=note> tags on this page, but the references will not show without a {{reflist|group=note}} template (see the help page).