
Back ثنائي نيوكليوتيد الفلافين والأدينين Arabic افایدی AZB Флавин-аденин-динуклеотид Bulgarian Flavin adenin-dinukleotid BS Dinucleòtid de flavina i adenina Catalan Flavinadenindinukleotid Czech Flavin-adenin-dinukleotid Danish Flavin-Adenin-Dinukleotid German Flavín adenín dinucleótido Spanish Flabina adenina dinukleotido Basque
| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| 3DMet | |
| 1208946 | |
| ChEBI | |
| ChEMBL | |
| DrugBank | |
| ECHA InfoCard | 100.005.149 |
| EC Number |
|
| 108834 | |
| KEGG | |
| MeSH | Flavin-Adenine+Dinucleotide |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
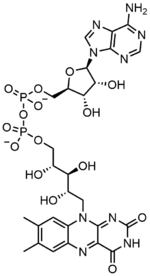
| C27H33N9O15P2 | |
| Molar mass | 785.557 g·mol−1 |
| Appearance | White, vitreous crystals |
| log P | -1.336 |
| Acidity (pKa) | 1.128 |
| Basicity (pKb) | 12.8689 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
In biochemistry, flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. A flavoprotein is a protein that contains a flavin group, which may be in the form of FAD or flavin mononucleotide (FMN). Many flavoproteins are known: components of the succinate dehydrogenase complex, α-ketoglutarate dehydrogenase, and a component of the pyruvate dehydrogenase complex.
FAD can exist in four redox states, which are the flavin-N(5)-oxide, quinone, semiquinone, and hydroquinone.[1] FAD is converted between these states by accepting or donating electrons. FAD, in its fully oxidized form, or quinone form, accepts two electrons and two protons to become FADH2 (hydroquinone form). The semiquinone (FADH·) can be formed by either reduction of FAD or oxidation of FADH2 by accepting or donating one electron and one proton, respectively. Some proteins, however, generate and maintain a superoxidized form of the flavin cofactor, the flavin-N(5)-oxide.[2][3]
- ^ Teufel, Robin; Agarwal, Vinayak; Moore, Bradley S. (2016-04-01). "Unusual flavoenzyme catalysis in marine bacteria". Current Opinion in Chemical Biology. 31: 31–39. doi:10.1016/j.cbpa.2016.01.001. ISSN 1879-0402. PMC 4870101. PMID 26803009.
- ^ Teufel, R; Miyanaga, A; Michaudel, Q; Stull, F; Louie, G; Noel, JP; Baran, PS; Palfey, B; Moore, BS (28 November 2013). "Flavin-mediated dual oxidation controls an enzymatic Favorskii-type rearrangement". Nature. 503 (7477): 552–6. Bibcode:2013Natur.503..552T. doi:10.1038/nature12643. PMC 3844076. PMID 24162851.
- ^ Teufel, Robin; Stull, Frederick; Meehan, Michael J.; Michaudel, Quentin; Dorrestein, Pieter C.; Palfey, Bruce; Moore, Bradley S. (2015-07-01). "Biochemical Establishment and Characterization of EncM's Flavin-N5-oxide Cofactor". Journal of the American Chemical Society. 137 (25): 8078–8085. doi:10.1021/jacs.5b03983. ISSN 1520-5126. PMC 4720136. PMID 26067765.