Back إيزوبرين Arabic İzopren Azerbaijani ایزوپرن AZB Ізапрэн Byelorussian Изопрен Bulgarian আইসোপ্রিন Bengali/Bangla Izopren BS Isoprè Catalan Isopren Czech Isopren Danish
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
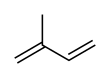
Isoprene
| |||
| Preferred IUPAC name
2-Methylbuta-1,3-diene | |||
| Other names
2-Methyl-1,3-butadiene
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.040 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H8 | |||
| Molar mass | 68.12 g/mol | ||
| Density | 0.681 g/cm3 | ||
| Melting point | −143.95 °C (−227.11 °F; 129.20 K) | ||
| Boiling point | 34.067 °C (93.321 °F; 307.217 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. It is produced by many plants and animals[1] (including humans) and its polymers are the main component of natural rubber. C. G. Williams named the compound in 1860 after obtaining it from the pyrolysis of natural rubber; he correctly deduced the empirical formula C5H8.[2][3]
- ^ Sharkey TD (1996). "Isoprene synthesis by plants and animals". Endeavour. 20 (2): 74–8. doi:10.1016/0160-9327(96)10014-4. PMID 8690002.
- ^ Williams CG (1860). "On isoprene and caoutchine". Proceedings of the Royal Society of London. 10: 516–519. doi:10.1098/rspl.1859.0101. S2CID 104233421.
- ^ Loadman MJ (2012-12-06). Analysis of Rubber and Rubber-like Polymers. Springer. p. 10. ISBN 9789401144353.