
Back ميوسين (بروتين) Arabic Миозин Bulgarian Miozin BS Miosina Catalan مایۆسین CKB Myosin Czech Myosin German Miosina Spanish Miosina Basque میوزین Persian
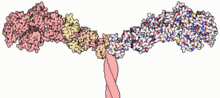
Myosins (/ˈmaɪəsɪn, -oʊ-/[1][2]) are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility.
The first myosin (M2) to be discovered was in 1864 by Wilhelm Kühne. Kühne had extracted a viscous protein from skeletal muscle that he held responsible for keeping the tension state in muscle. He called this protein myosin.[3][4] The term has been extended to include a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue.
Following the discovery in 1973 of enzymes with myosin-like function in Acanthamoeba castellanii, a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes.[5]
Although myosin was originally thought to be restricted to muscle cells (hence myo-(s) + -in), there is no single "myosin"; rather it is a very large superfamily of genes whose protein products share the basic properties of actin binding, ATP hydrolysis (ATPase enzyme activity), and force transduction. Virtually all eukaryotic cells contain myosin isoforms. Some isoforms have specialized functions in certain cell types (such as muscle), while other isoforms are ubiquitous. The structure and function of myosin is globally conserved across species, to the extent that rabbit muscle myosin II will bind to actin from an amoeba.[6]
- ^ "Myosin". Merriam-Webster.com Dictionary.
- ^ "myosin - definition of myosin in English from the Oxford dictionary". OxfordDictionaries.com. Archived from the original on 24 August 2012. Retrieved 20 January 2016.
- ^ Hartman, MA; Spudich, JA (1 April 2012). "The myosin superfamily at a glance". Journal of Cell Science. 125 (Pt 7): 1627–32. doi:10.1242/jcs.094300. PMC 3346823. PMID 22566666.
- ^ Szent-Györgyi, AG (June 2004). "The early history of the biochemistry of muscle contraction". The Journal of General Physiology. 123 (6): 631–41. doi:10.1085/jgp.200409091. PMC 2234565. PMID 15173217.
- ^ Pollard TD, Korn ED (July 1973). "Acanthamoeba myosin. I. Isolation from Acanthamoeba castellanii of an enzyme similar to muscle myosin". The Journal of Biological Chemistry. 248 (13): 4682–90. doi:10.1016/S0021-9258(19)43718-6. PMID 4268863. Archived from the original on 6 January 2016.
- ^ McMahon, T. A. 1984. Muscles, Reflexes and Locomotion. 1st Edition. Princeton University Press. ISBN 978-0-691-02376-2