
Back Skeletformule Afrikaans صيغة هيكلية Arabic Skeletna formula BS Fórmula esquelètica Catalan Skeletformel Danish Skelettformel German Fórmula esqueletal Spanish Graafiline struktuurivalem Estonian فرمول اسکلتی Persian Viivakaava Finnish
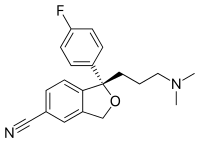
The skeletal formula, line-angle formula, bond-line formula or shorthand formula of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecule's bonding and some details of its molecular geometry. A skeletal formula shows the skeletal structure or skeleton of a molecule, which is composed of the skeletal atoms that make up the molecule.[1] It is represented in two dimensions, as on a piece of paper. It employs certain conventions to represent carbon and hydrogen atoms, which are the most common in organic chemistry.
An early form of this representation was first developed by organic chemist August Kekulé, while the modern form is closely related to and influenced by the Lewis structure of molecules and their valence electrons. Hence they are sometimes termed Kekulé structures[a] or Lewis–Kekulé structures. Skeletal formulae have become ubiquitous in organic chemistry, partly because they are relatively quick and simple to draw, and also because the curved arrow notation used for discussions of reaction mechanisms and electron delocalization can be readily superimposed.
Several other ways of depicting chemical structures are also commonly used in organic chemistry (though less frequently than skeletal formulae). For example, conformational structures look similar to skeletal formulae and are used to depict the approximate positions of atoms in 3D space, as a perspective drawing. Other types of representation, such as Newman projection, Haworth projection or Fischer projection, also look somewhat similar to skeletal formulae. However, there are slight differences in the conventions used, and the reader needs to be aware of them in order to understand the structural details encoded in the depiction. While skeletal and conformational structures are also used in organometallic and inorganic chemistry, the conventions employed also differ somewhat.
- ^ Stoker, H. Stephen (2012). General, Organic, and Biological Chemistry (6th ed.). Cengage. ISBN 978-1133103943.[page needed]
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).