
Back Benzoin-Addition German Condensación benzoínica Spanish Bensoiinkondensatsioon Estonian تراکم بنزوئین Persian Bentsoiinikondensaatio Finnish Condensation benzoïnique French Condensazione benzoinica Italian ベンゾイン縮合 Japanese Benzoïnecondensatie Dutch Condensare benzoinică Romanian
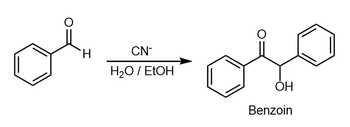
In organic chemistry, the benzoin addition is an addition reaction involving two aldehydes (−CH=O). The reaction generally occurs between aromatic aldehydes or glyoxals (OCH=CHO),[1][2] and results in formation of an acyloin (−C(O)CH(OH)−). In the classic example, benzaldehyde is converted to benzoin (PhCH(OH)C(O)Ph).[3]
The benzoin condensation was first reported in 1832 by Justus von Liebig and Friedrich Wöhler during their research on bitter almond oil.[4] The catalytic version of the reaction involving cyanide was developed by Nikolay Zinin in the late 1830s.[5][6]
- ^ Menon, Rajeev S.; Biju, Akkattu T.; Nair, Vijay (2016). "Recent advances in N-heterocyclic carbene (NHC)-catalysed benzoin reactions". Beilstein Journal of Organic Chemistry. 12: 444–461. doi:10.3762/bjoc.12.47. PMC 4901930. PMID 27340440.
- ^ Enders, Dieter; Niemeier, Oliver; Henseler, Alexander (2007). "Organocatalysis by N-Heterocyclic Carbenes". Chemical Reviews. 107 (12): 5606–5655. doi:10.1021/cr068372z. PMID 17956132.
- ^ Roger Adams; C. S. Marvel (1921). "Benzoin". Organic Syntheses. 1: 33. doi:10.15227/orgsyn.001.0033.
- ^ F. Wöhler, J. Liebig (1832). "Untersuchungen über das Radikal der Benzoesäure" [Studies on the radicals of benzoic acid]. Annalen der Pharmacie (in German). 3 (3): 249–282. doi:10.1002/jlac.18320030302. hdl:2027/hvd.hxdg3f.
- ^ N. Zinin (1839). "Beiträge zur Kenntniss einiger Verbindungen aus der Benzoylreihe" [Contributions to the knowledge of some compounds from the benzoyl series]. Annalen der Pharmacie (in German). 31 (3): 329–332. doi:10.1002/jlac.18390310312. Archived from the original on 2022-07-09. Retrieved 2020-09-11.
- ^ N. Zinin (1840). "Ueber einige Zersetzungsprodukte des Bittermandelöls" [Study of some decomposition products of bitter almond oil]. Annalen der Pharmacie (in German). 34 (2): 186–192. doi:10.1002/jlac.18400340205. Archived from the original on 2022-07-09. Retrieved 2019-06-28.