Back تفاعل كانيزارو Arabic Рэакцыя Каніцара Byelorussian Рэакцыя Каніцара BE-X-OLD Реакция на Каницаро Bulgarian ক্যানিজারো বিক্রিয়া Bengali/Bangla Reacció de Cannizzaro Catalan Cannizzarova reakce Czech Cannizzaro-Reaktion German Reacción de Cannizzaro Spanish Canizzaroren erreakzio Basque
| Cannizzaro reaction | |
|---|---|
| Named after | Stanislao Cannizzaro |
| Reaction type | Organic redox reaction |
| Identifiers | |
| Organic Chemistry Portal | cannizzaro-reaction |
| RSC ontology ID | RXNO:0000218 |
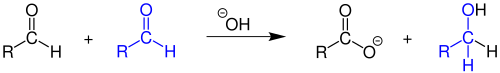
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction which involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to give a primary alcohol and a carboxylic acid.[1][2]
Cannizzaro first accomplished this transformation in 1853, when he obtained benzyl alcohol and potassium benzoate from the treatment of benzaldehyde with potash (potassium carbonate). More typically, the reaction would be conducted with sodium hydroxide or potassium hydroxide, giving the sodium or potassium carboxylate salt of the carboxylic-acid product:
- 2 C6H5CHO + KOH → C6H5CH2OH + C6H5COOK
The process is a redox reaction involving transfer of a hydride from one substrate molecule to the other: one aldehyde is oxidized to form the acid, the other is reduced to form the alcohol.[3]
- ^ Cannizzaro, S. (1853). "Ueber den der Benzoësäure entsprechenden Alkohol" [On the alcohol corresponding to benzoic acid]. Liebigs Annalen der Chemie und Pharmacie. 88: 129–130. doi:10.1002/jlac.18530880114.
- ^ List, K.; Limpricht, H. (1854). "Ueber das sogenannte Benzoëoxyd und einige andere gepaarte Verbindungen" [On so-called benzoic oxide and some other paired compounds]. Liebigs Annalen der Chemie und Pharmacie. 90 (2): 190–210. doi:10.1002/jlac.18540900211.
- ^ Geissman, T. A. "The Cannizzaro Reaction" Org. React. 1944, 2, 94. doi:10.1002/0471264180.or002.03(Review)