
Back سيفيوروكزيم Arabic سفوروکسیم AZB সেফুরোক্সিম Bengali/Bangla Cefuroxima Catalan Ceffwrocsim Welsh Cefuroxim German Κεφουροξίμη Greek Cefuroxima Spanish Zefuroxima Basque سفوروکسیم Persian
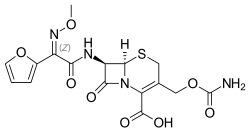 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zinacef, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601206 |
| License data |
|
| Routes of administration | Intramuscular, intravenous, by mouth |
| Drug class | Second-generation cephalosporin |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 37% on an empty stomach, up to 52% if taken after food |
| Elimination half-life | 80 minutes |
| Excretion | Urine 66–100% unchanged |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.054.127 |
| Chemical and physical data | |
| Formula | C16H16N4O8S |
| Molar mass | 424.38 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cefuroxime, sold under the brand name Zinacef among others, is a second-generation cephalosporin[2] antibiotic used to treat and prevent a number of bacterial infections.[3] These include pneumonia, meningitis, otitis media, sepsis, urinary tract infections, and Lyme disease.[4] It is used by mouth or by injection into a vein or muscle.[4]
Common side effects include nausea, diarrhea, allergic reactions, and pain at the site of injection.[4] Serious side effects may include Clostridioides difficile infection, anaphylaxis, and Stevens–Johnson syndrome.[4] Use in pregnancy and breastfeeding is believed to be safe.[5] It is a second-generation cephalosporin and works by interfering with a bacteria's ability to make a cell wall resulting in its death.[4]
Cefuroxime was patented in 1971 and approved for medical use in 1977.[6] It is on the World Health Organization's List of Essential Medicines.[7] In 2022, it was the 283rd most commonly prescribed medication in the United States, with more than 600,000 prescriptions.[8][9]
- ^ "Product monograph brand safety updates". Health Canada. 7 July 2016. Retrieved 13 July 2024.
- ^ Katzung B (2018). Basic & Clinical Pharmacology. McGraw Hill. p. 803.
- ^ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 518. ISBN 9780857113382.
- ^ a b c d e "Cefuroxime Sodium Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 22 March 2019.
- ^ "Cefuroxime Use During Pregnancy". Drugs.com. Retrieved 3 March 2019.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 493. ISBN 9783527607495.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Cefuroxime Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.