
Back Enantiomeer Afrikaans مصاوغ مرآتي Arabic Енантиомер Bulgarian Enantiomer BS Enantiòmer Catalan Enantiomer Czech Enantiomer Danish Enantiomer German Εναντιομερές Greek Enantiomero Esperanto
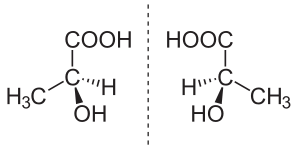
In chemistry, an enantiomer (/ɪˈnænti.əmər, ɛ-, -oʊ-/[1] ih-NAN-tee-ə-mər), also known as an optical isomer,[2] antipode,[3] or optical antipode,[4] is one of a pair of molecular entities which are mirror images of each other and non-superposable.
Enantiomer molecules are like right and left hands: one cannot be superposed onto the other without first being converted to its mirror image.[5] It is solely a relationship of chirality and the permanent three-dimensional relationships among molecules or other chemical structures: no amount of re-orientiation of a molecule as a whole or conformational change converts one chemical into its enantiomer. Chemical structures with chirality rotate plane-polarized light.[6] A mixture of equal amounts of each enantiomer, a racemic mixture or a racemate, does not rotate light.[7][8][9]
Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are also nonsuperposable onto each other; however, they are not mirror images of each other.[10]
- ^ "Enantiomer: Definition & Meaning". Dictionary.com. Retrieved 2024-04-26.
- ^ Chemistry (IUPAC), The International Union of Pure and Applied. "IUPAC - optical isomers (O04308)". goldbook.iupac.org. doi:10.1351/goldbook.O04308. Retrieved 2022-11-17.
- ^ Chemistry (IUPAC), The International Union of Pure and Applied. "IUPAC - antipodes (A00403)". goldbook.iupac.org. doi:10.1351/goldbook.A00403. Retrieved 2022-11-17.
- ^ Chemistry (IUPAC), The International Union of Pure and Applied. "IUPAC - optical antipodes (O04304)". goldbook.iupac.org. doi:10.1351/goldbook.O04304. Retrieved 2022-11-17.
- ^ McConathy, Jonathan; Owens, Michael J. (2003). "Stereochemistry in Drug Action". Primary Care Companion to the Journal of Clinical Psychiatry. 5 (2): 70–73. doi:10.4088/pcc.v05n0202. ISSN 1523-5998. PMC 353039. PMID 15156233.
- ^ "Chirality and Optical Activity". chemed.chem.purdue.edu. Retrieved 2022-11-17.
- ^ Chemistry (IUPAC), The International Union of Pure and Applied. "IUPAC - racemic (R05026)". goldbook.iupac.org. doi:10.1351/goldbook.R05026. Retrieved 2022-11-17.
- ^ Chemistry (IUPAC), The International Union of Pure and Applied. "IUPAC - racemate (R05025)". goldbook.iupac.org. doi:10.1351/goldbook.R05025. Retrieved 2022-11-17.
- ^ Weber, Erin. "Library Guides: CHEM 221: Stereochemistry / Isomerism". libraryguides.salisbury.edu. Retrieved 2022-11-17.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1