Back Metamfetamien Afrikaans ميثامفيتامين Arabic Metamfetamin Azerbaijani متامفتامین AZB Shabu BCL Мэтамфэтамін BE-X-OLD Метамфетамин Bulgarian মেথামফেটামিন Bengali/Bangla Metamfetamin BS Metamfetamina Catalan
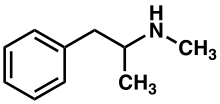
Methamphetamine[note 1] (contracted from N-methylamphetamine) is a potent central nervous system (CNS) stimulant that is mainly used as a recreational or performance-enhancing drug and less commonly as a second-line treatment for attention deficit hyperactivity disorder (ADHD).[23] It has also been researched as a potential treatment for traumatic brain injury.[7] Methamphetamine was discovered in 1893 and exists as two enantiomers: levo-methamphetamine and dextro-methamphetamine.[note 2] Methamphetamine properly refers to a specific chemical substance, the racemic free base, which is an equal mixture of levomethamphetamine and dextromethamphetamine in their pure amine forms, but the hydrochloride salt, commonly called crystal meth, is widely used. Methamphetamine is rarely prescribed over concerns involving its potential for recreational use as an aphrodisiac and euphoriant, among other concerns, as well as the availability of safer substitute drugs with comparable treatment efficacy such as Adderall and Vyvanse.[23] While pharmaceutical formulations of methamphetamine in the United States are labeled as methamphetamine hydrochloride, they contain dextromethamphetamine as the active ingredient.[23][note 3] Dextromethamphetamine is a stronger CNS stimulant than levomethamphetamine.[23]
Both racemic methamphetamine and dextromethamphetamine are illicitly trafficked and sold owing to their potential for recreational use. The highest prevalence of illegal methamphetamine use occurs in parts of Asia and Oceania, and in the United States, where racemic methamphetamine and dextromethamphetamine are classified as Schedule II controlled substances. Levomethamphetamine is available as an over-the-counter (OTC) drug for use as an inhaled nasal decongestant in the United States.[note 4] Internationally, the production, distribution, sale, and possession of methamphetamine is restricted or banned in many countries, owing to its placement in schedule II of the United Nations Convention on Psychotropic Substances treaty. While dextromethamphetamine is a more potent drug, racemic methamphetamine is illicitly produced more often, owing to the relative ease of synthesis and regulatory limits of chemical precursor availability.
In low to moderate doses, methamphetamine can elevate mood, increase alertness, concentration and energy in fatigued individuals, reduce appetite, and promote weight loss. At very high doses, it can induce psychosis, breakdown of skeletal muscle, seizures, and bleeding in the brain. Chronic high-dose use can precipitate unpredictable and rapid mood swings, stimulant psychosis (e.g., paranoia, hallucinations, delirium, and delusions), and violent behavior. Recreationally, methamphetamine's ability to increase energy has been reported to lift mood and increase sexual desire to such an extent that users are able to engage in sexual activity continuously for several days while binging the drug.[28] Methamphetamine is known to possess a high addiction liability (i.e., a high likelihood that long-term or high dose use will lead to compulsive drug use) and high dependence liability (i.e., a high likelihood that withdrawal symptoms will occur when methamphetamine use ceases). Discontinuing methamphetamine after heavy use may lead to a post-acute-withdrawal syndrome, which can persist for months beyond the typical withdrawal period. At high doses, methamphetamine is neurotoxic to human midbrain dopaminergic neurons and, to a lesser extent, serotonergic neurons.[29][30] Methamphetamine neurotoxicity causes adverse changes in brain structure and function, such as reductions in grey matter volume in several brain regions, as well as adverse changes in markers of metabolic integrity.[30]
Methamphetamine belongs to the substituted phenethylamine and substituted amphetamine chemical classes. It is related to the other dimethylphenethylamines as a positional isomer of these compounds, which share the common chemical formula C10H15N.
- ^ "methamphetamine". Methamphetamine. Lexico. Archived from the original on 14 June 2021. Retrieved 22 April 2022.
- ^ Anvisa (24 July 2023). "RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 25 July 2023). Archived from the original on 27 August 2023. Retrieved 27 August 2023.
- ^ Ingersoll J (7 July 1971). "Amphetamine, Methamphetamine, and Optical Isomers" (PDF). Federal Register. Bureau of Narcotics and Dangerous Drugs. Archived (PDF) from the original on 27 November 2024. Retrieved 27 November 2024.
- ^ a b c d e f g h i Cite error: The named reference
pmid19426289was invoked but never defined (see the help page). - ^ a b c d e Cite error: The named reference
Schepwas invoked but never defined (see the help page). - ^ a b c Courtney KE, Ray LA (October 2014). "Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature". Drug Alcohol Depend. 143: 11–21. doi:10.1016/j.drugalcdep.2014.08.003. PMC 4164186. PMID 25176528.
- ^ a b c Rau T, Ziemniak J, Poulsen D (January 2016). "The neuroprotective potential of low-dose methamphetamine in preclinical models of stroke and traumatic brain injury". Progress in Neuro-psychopharmacology & Biological Psychiatry. 64: 231–236. doi:10.1016/j.pnpbp.2015.02.013. ISSN 0278-5846. PMID 25724762.
- ^ "Methamphetamine: Toxicity". PubChem Compound. National Center for Biotechnology Information. Archived from the original on 4 January 2015. Retrieved 4 January 2015.
- ^ Sellers EM, Tyndale RF (2000). "Mimicking gene defects to treat drug dependence". Ann. N. Y. Acad. Sci. 909 (1): 233–246. Bibcode:2000NYASA.909..233S. doi:10.1111/j.1749-6632.2000.tb06685.x. PMID 10911933. S2CID 27787938.
Methamphetamine, a central nervous system stimulant drug, is p-hydroxylated by CYP2D6 to less active p-OH-methamphetamine.
- ^ Cite error: The named reference
FDA Pharmacokineticswas invoked but never defined (see the help page). - ^ Cite error: The named reference
FMOwas invoked but never defined (see the help page). - ^ Cite error: The named reference
FMO3-Primarywas invoked but never defined (see the help page). - ^ a b "Methamphetamine: Chemical and Physical Properties". PubChem Compound. National Center for Biotechnology Information. Archived from the original on 4 January 2015. Retrieved 4 January 2015.
- ^ a b "Methamphetamine". Drug profiles. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). 8 January 2015. Archived from the original on 15 April 2016. Retrieved 27 November 2018.
The term metamfetamine (the International Non-Proprietary Name: INN) strictly relates to the specific enantiomer (S)-N,α-dimethylbenzeneethanamine.
- ^ "Methamphetamine: Identification". DrugBank. University of Alberta. 8 February 2013. Archived from the original on 28 December 2015. Retrieved 1 January 2014.
- ^ "Methedrine (methamphetamine hydrochloride): Uses, Symptoms, Signs and Addiction Treatment". Addictionlibrary.org. Archived from the original on 4 March 2016. Retrieved 16 January 2016.
- ^ "Polisi Tangkap Bandar Shabu-shabu". Detik News (in Indonesian). Archived from the original on 29 July 2023. Retrieved 29 July 2023.
- ^ "P1-M shabu seized from 3 drug pushers". Manila Bulletin. Retrieved 29 July 2023.
- ^ "Jadi pengedar sabu seorang IRT di Pidoli Dolok ditangkap Polisi – ANTARA News Sumatera Utara". ANTARA News Agency. Archived from the original on 22 September 2024. Retrieved 29 July 2023.
- ^ Marantal RD. "E-bike driver nabbed in drug bust, shabu worth almost P1 million seized". Philstar.com. Archived from the original on 29 July 2023. Retrieved 29 July 2023.
- ^ "Meth Slang Names". MethhelpOnline. Archived from the original on 7 December 2013. Retrieved 1 January 2014.
- ^ "Methamphetamine and the law". Archived from the original on 28 January 2015. Retrieved 30 December 2014.
- ^ a b c d Moszczynska A, Callan SP (September 2017). "Molecular, Behavioral, and Physiological Consequences of Methamphetamine Neurotoxicity: Implications for Treatment". The Journal of Pharmacology and Experimental Therapeutics. 362 (3): 474–488. doi:10.1124/jpet.116.238501. PMC 11047030. PMID 28630283.
METH is a schedule II drug, which can only be prescribed for attention deficit hyperactivity disorder (ADHD), extreme obesity, or narcolepsy (as Desoxyn; Recordati Rare Diseases LLC, Lebanon, NJ), with amphetamine being prescribed more often for these conditions due to amphetamine having lower reinforcing potential than METH (Lile et al., 2013). ...
As discussed earlier, the d-enantiomer has stronger CNS effects but is metabolized more quickly than the l-enantiomer, which is longer lasting due to the slower breakdown. ...
l-METH, a vasoconstrictor, is the active constituent of the Vicks Inhaler decongestant (Proctor & Gamble, Cincinnati, OH), an over-the-counter product containing about 50 mg of the drug (Smith et al., 2014). Desoxyn, which is d-METH, is rarely medically prescribed due to its strong reinforcing properties. Therapeutic doses of Desoxyn are 20–25 mg daily, taken every 12 hours, with dosing not exceeding 60 mg/day - ^ "Levomethamphetamine". Pubchem Compound. National Center for Biotechnology Information. Archived from the original on 6 October 2014. Retrieved 27 November 2018.
- ^ Cite error: The named reference
Desoxyn FDA labelwas invoked but never defined (see the help page). - ^ "Code of Federal Regulations Title 21: Subchapter D – Drugs for human use, Part 341 – cold, cough, allergy, bronchodilator, and antiasthmatic drug products for over-the-counter human use". United States Food and Drug Administration. April 2015. Archived from the original on 25 December 2019. Retrieved 7 March 2016.
Topical nasal decongestants --(i) For products containing levmetamfetamine identified in 341.20(b)(1) when used in an inhalant dosage form. The product delivers in every 800 milliliters of air 0.04 to 0.150 milligrams of levmetamfetamine.
- ^ "Levomethamphetamine: Identification". Pubchem Compound. National Center for Biotechnology Information. Archived from the original on 6 October 2014. Retrieved 4 September 2017.
- ^ "Meth's aphrodisiac effect adds to drug's allure". NBC News. Associated Press. 3 December 2004. Archived from the original on 12 August 2013. Retrieved 12 September 2019.
- ^ Yu S, Zhu L, Shen Q, Bai X, Di X (March 2015). "Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology". Behavioural Neurology. 2015 (103969): 1–11. doi:10.1155/2015/103969. PMC 4377385. PMID 25861156.
- ^ a b Cite error: The named reference
pmid19328213was invoked but never defined (see the help page).
Cite error: There are <ref group=note> tags on this page, but the references will not show without a {{reflist|group=note}} template (see the help page).