
Back مراجعة منهجية Arabic Revisió sistemàtica Catalan Systematische Übersichtsarbeit German Συστηματική ανασκόπηση Greek Revisión sistemática Spanish مرور سیستماتیک Persian Systemaattinen kirjallisuuskatsaus Finnish Revue systématique French Szisztematikus áttekintés Hungarian Tinjauan sistematis ID
| Part of a series on |
| Research |
|---|
 |
| Philosophy portal |
A systematic review is a scholarly synthesis of the evidence on a clearly presented topic using critical methods to identify, define and assess research on the topic.[1] A systematic review extracts and interprets data from published studies on the topic (in the scientific literature), then analyzes, describes, critically appraises and summarizes interpretations into a refined evidence-based conclusion.[1][2] For example, a systematic review of randomized controlled trials is a way of summarizing and implementing evidence-based medicine.[3] Systematic reviews, sometimes along with meta-analyses, are generally considered the highest level of evidence in medical research.[4][5]
While a systematic review may be applied in the biomedical or health care context, it may also be used where an assessment of a precisely defined subject can advance understanding in a field of research.[6] A systematic review may examine clinical tests, public health interventions, environmental interventions,[7] social interventions, adverse effects, qualitative evidence syntheses, methodological reviews, policy reviews, and economic evaluations.[8][9]
Systematic reviews are closely related to meta-analyses, and often the same instance will combine both (being published with a subtitle of "a systematic review and meta-analysis"). The distinction between the two is that a meta-analysis uses statistical methods to induce a single number from the pooled data set (such as an effect size), whereas the strict definition of a systematic review excludes that step. However, in practice, when one is mentioned, the other may often be involved, as it takes a systematic review to assemble the information that a meta-analysis analyzes, and people sometimes refer to an instance as a systematic review, even if it includes the meta-analytical component.
An understanding of systematic reviews and how to implement them in practice is common for professionals in health care, public health, and public policy.[1]
Systematic reviews contrast with a type of review often called a narrative review. Systematic reviews and narrative reviews both review the literature (the scientific literature), but the term literature review without further specification refers to a narrative review.
- ^ a b c "What is a systematic review?". Temple University Libraries. 6 June 2022. Retrieved 15 June 2022.
- ^ Armstrong R, Hall BJ, Doyle J, Waters E (March 2011). "Cochrane Update. 'Scoping the scope' of a cochrane review". Journal of Public Health. 33 (1): 147–150. doi:10.1093/pubmed/fdr015. PMID 21345890.
- ^ "What is EBM?". Centre for Evidence Based Medicine. 20 November 2009. Archived from the original on 6 April 2011. Retrieved 17 June 2011.
- ^ Wallace, Sowdhamini S.; Barak, Gal; Truong, Grace; Parker, Michelle W. (1 August 2022). "Hierarchy of Evidence Within the Medical Literature". Hospital Pediatrics. 12 (8): 745. doi:10.1542/hpeds.2022-006690.
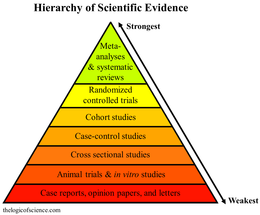
The quality of evidence from medical research is partially deemed by the hierarchy of study designs. On the lowest level, the hierarchy of study designs begins with animal and translational studies and expert opinion, and then ascends to descriptive case reports or case series, followed by analytic observational designs such as cohort studies, then randomized controlled trials, and finally systematic reviews and meta-analyses as the highest quality evidence.
- ^ Murad, M Hassan; Asi, Noor; Alsawas, Mouaz; Alahdab, Fares (August 2016). "New evidence pyramid". Evidence Based Medicine. 21 (4): 125. doi:10.1136/ebmed-2016-110401.
A pyramid has expressed the idea of hierarchy of medical evidence for so long, that not all evidence is the same. Systematic reviews and meta-analyses have been placed at the top of this pyramid for several good reasons.
- ^ Ader HJ, Mellenbergh GJ, Hand DJ (2008). "Methodological quality". Advising on Research Methods: A consultant's companion. Johannes van Kessel Publishing. ISBN 978-90-79418-02-2.
- ^ Bilotta GS, Milner AM, Boyd I (2014). "On the use of systematic reviews to inform environmental policies". Environmental Science & Policy. 42: 67–77. Bibcode:2014ESPol..42...67B. doi:10.1016/j.envsci.2014.05.010.
- ^ Systematic reviews: CRD's guidance for undertaking reviews in health care (PDF). York: University of York, Centre for Reviews and Dissemination. 2008. ISBN 978-1-900640-47-3. Retrieved 17 June 2011.
- ^ Petticrew M, Roberts H (2006). Systematic reviews in the social sciences (PDF). Wiley Blackwell. ISBN 978-1-4051-2110-1. Archived from the original (PDF) on 16 June 2015.