
Back Proton Afrikaans Proton ALS Protón AN प्रोटॉन ANP بروتون Arabic پروطون ARY প্ৰ'টন Assamese Protón AST Proton (fizika) Azerbaijani پروتون AZB
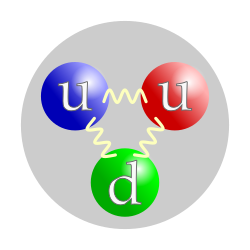 The valence quark content of a proton. The color assignment of individual quarks is arbitrary, but all three colors must be present. Forces between quarks are mediated by gluons. | |
| Classification | Baryon |
|---|---|
| Composition | 2 up quarks (u), 1 down quark (d) |
| Statistics | Fermionic |
| Family | Hadron |
| Interactions | Gravity, electromagnetic, weak, strong |
| Symbol | p, p+ , N+ , 1 1H+ |
| Antiparticle | Antiproton |
| Theorized | William Prout (1815) |
| Discovered | Observed as H+ by Eugen Goldstein (1886). Identified in other nuclei (and named) by Ernest Rutherford (1917–1920). |
| Mass | 1.67262192595(52)×10−27 kg[1] 1.0072764665789(83) Da[2] 938.27208943(29) MeV/c2[3] |
| Mean lifetime | > 3.6×1029 years[4] (stable) |
| Electric charge | +1 e |
| Charge radius | 0.8414(19) fm[5] |
| Electric dipole moment | < 2.1×10−25 e⋅cm[6] |
| Electric polarizability | 0.00112(4) fm3 |
| Magnetic moment | 1.41060679545(60)×10−26 J⋅T−1[7] 0.00152103220230(45) μB[8] 2.79284734463(82) μN[9] |
| Magnetic polarizability | 1.9(5)×10−4 fm3 |
| Spin | 1/2 ħ |
| Isospin | 1/2 |
| Parity | +1 |
| Condensed | I(JP) = 1/2(1/2+) |
A proton is a stable subatomic particle, symbol p, H+, or 1H+ with a positive electric charge of +1 e (elementary charge). Its mass is slightly less than the mass of a neutron and approximately 1836 times the mass of an electron (the proton-to-electron mass ratio). Protons and neutrons, each with a mass of approximately one dalton, are jointly referred to as nucleons (particles present in atomic nuclei).
One or more protons are present in the nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol Z). Since each element is identified by the number of protons in its nucleus, each element has its own atomic number, which determines the number of atomic electrons and consequently the chemical characteristics of the element.
The word proton is Greek for "first", and the name was given to the hydrogen nucleus by Ernest Rutherford in 1920. In previous years, Rutherford had discovered that the hydrogen nucleus (known to be the lightest nucleus) could be extracted from the nuclei of nitrogen by atomic collisions.[10] Protons were therefore a candidate to be a fundamental or elementary particle, and hence a building block of nitrogen and all other heavier atomic nuclei.
Although protons were originally considered to be elementary particles, in the modern Standard Model of particle physics, protons are known to be composite particles, containing three valence quarks, and together with neutrons are now classified as hadrons. Protons are composed of two up quarks of charge +2/3e each, and one down quark of charge −1/3e. The rest masses of quarks contribute only about 1% of a proton's mass.[11] The remainder of a proton's mass is due to quantum chromodynamics binding energy, which includes the kinetic energy of the quarks and the energy of the gluon fields that bind the quarks together. The proton charge radius is around 0.841 fm but two different kinds of measurements give slightly different values.[12]
At sufficiently low temperatures and kinetic energies, free protons will bind electrons in any matter they traverse. Free protons are routinely used for accelerators for proton therapy or various particle physics experiments, with the most powerful example being the Large Hadron Collider.
- ^ "2022 CODATA Value: proton mass". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 2024-05-18.
- ^ "2022 CODATA Value: proton mass in u". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 2024-05-18.
- ^ "2022 CODATA Value: proton mass energy equivalent in MeV". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 2024-05-18.
- ^ The SNO+ Collaboration; Anderson, M.; Andringa, S.; Arushanova, E.; Asahi, S.; Askins, M.; Auty, D. J.; Back, A. R.; Barnard, Z.; Barros, N.; Bartlett, D. (2019-02-20). "Search for invisible le modes of nucleon decay in water with the SNO+ detector". Physical Review D. 99 (3): 032008. arXiv:1812.05552. Bibcode:2019PhRvD..99c2008A. doi:10.1103/PhysRevD.99.032008. S2CID 96457175.
- ^ Cite error: The named reference
CODATA2018was invoked but never defined (see the help page). - ^ Sahoo, B. K. (2017-01-17). "Improved limits on the hadronic and semihadronic $CP$ violating parameters and role of a dark force carrier in the electric dipole moment of $^{199}\mathrm{Hg}$". Physical Review D. 95 (1): 013002. arXiv:1612.09371. doi:10.1103/PhysRevD.95.013002. S2CID 119344894.
- ^ "2022 CODATA Value: proton magnetic moment". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 2024-05-18.
- ^ "2022 CODATA Value: proton magnetic moment to Bohr magneton ratio". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 2024-05-18.
- ^ "2022 CODATA Value: proton magnetic moment to nuclear magneton ratio". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 2024-05-18.
- ^ Cite error: The named reference
Britannicawas invoked but never defined (see the help page). - ^ Cite error: The named reference
Masswas invoked but never defined (see the help page). - ^ N Baryons S. Navas et al. (Particle Data Group), Phys. Rev. D 110, 030001 (2024) and 2025 update