Electrical Safety | KS2 Science | STEM and Beyond Do you like learning about electricity? Let's look at electrical safety! In this video, we will find out more about the dangers of ...
Show Comments
Public Health England - Activity on GOV.UK
Medicines and Healthcare products Regulatory Agency - Activity on GOV.UK
Electricity! ⚡ Electrical Safety with The Wiggles and Queensland Electrical Safety Office The Wiggles have partnered with the Queensland Government's Electrical Safety Office to deliver life-saving electrical safety ...
Medicines and Healthcare products Regulatory Agency - Activity on GOV.UK
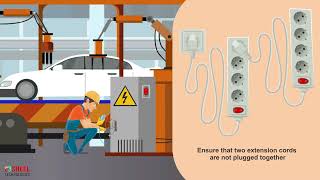
10 Electrical Safety Rules Training Video in English || Hazards & Precautions #electricalsafety Electrically powered equipment can pose a significant hazard to workers, particularly when mishandled or not maintained.
Preventing Workplace Hazards: Basic Electrical Safety Training Electricity powers our world, but without proper precautions, it can be a silent and deadly threat. Each year, thousands of ...
Public Health England - Activity on GOV.UK
Public Health England - Activity on GOV.UK
OSHA Electrical Safety: How To Prevent Electrical Hazards. Automate Safety Talks and Save Time - https://safelyio.com/ Generate Free safety toolbox talks PDFs ...
Public Health England - Activity on GOV.UK
Public Health England - Activity on GOV.UK
Public Health England - Activity on GOV.UK
Public Health England - Activity on GOV.UK
Electrical Safety Tips: Avoid These Dangerous Practices! Welcome to our latest safety video on electrical safety! Understanding and avoiding electrical hazards can save lives. In this video ...
Arc Flash and Electrical Safety: Safe Work Practices Implementing the appropriate safety measures and safe work practices may make the difference between life and death when ...
Load More